Magnesium Glycinate Powder
Your order will be shipped tomorrow
Benefits
Time to notice full effects: 2-3 Weeks (With Immediate Effects)
-
Sleep
-
Cognition
-
Stress
-
Mood
Summary
Magnesium Glycinate is a vital mineral supplement essential for over 300 enzyme systems. It may improve sleep quality, support stress management, and enhance cognitive function.
Product Usage
1600mg (Per Serving)
Night
With Or Without Food
1 Time Daily
Build Your Stack
Unlock your full potential by building a supplement stack tailored to your unique goals. Whether you're focused on energy, focus, or overall wellness, we’re here to guide you in creating the perfect combination of supplements to support your journey. Your health, your way—powered by the right stack.

Magnesium Glycinate General Overview
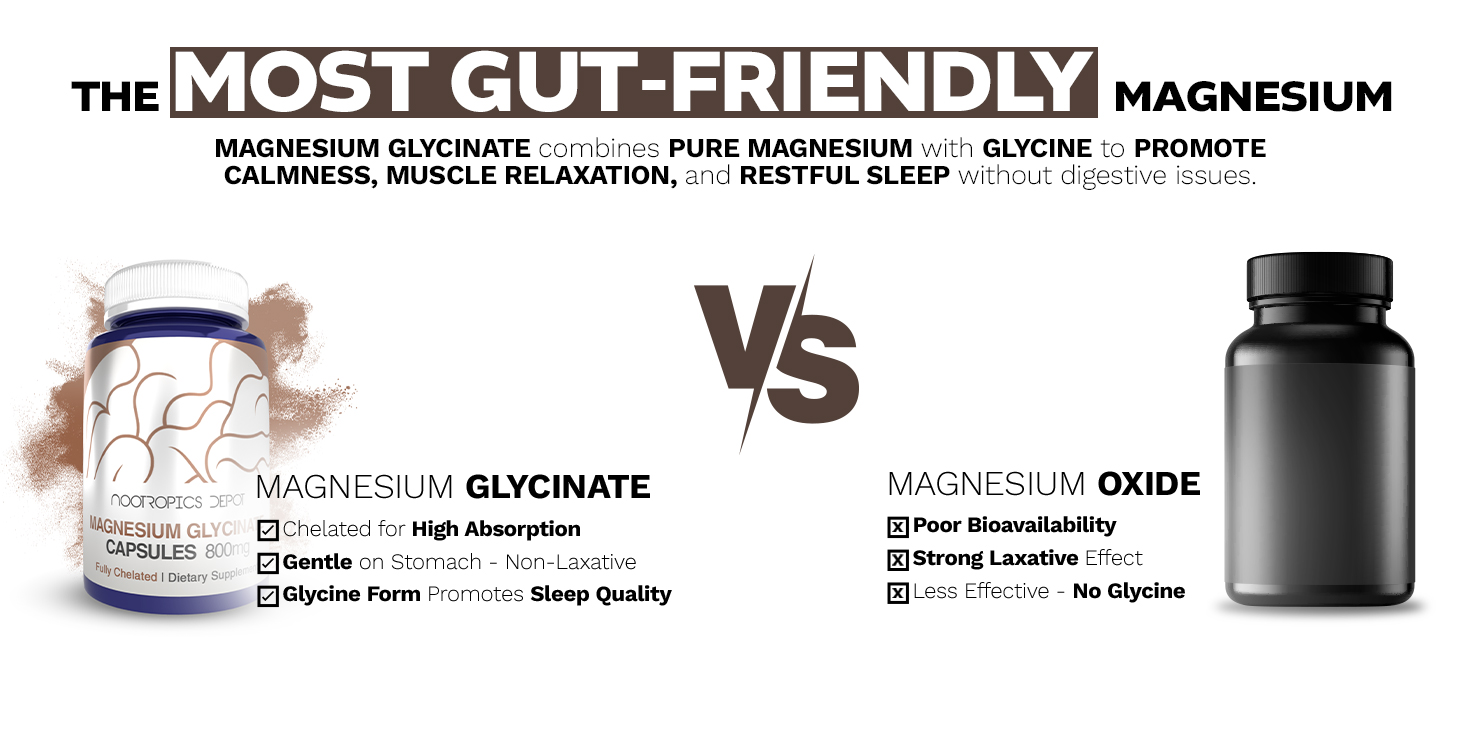
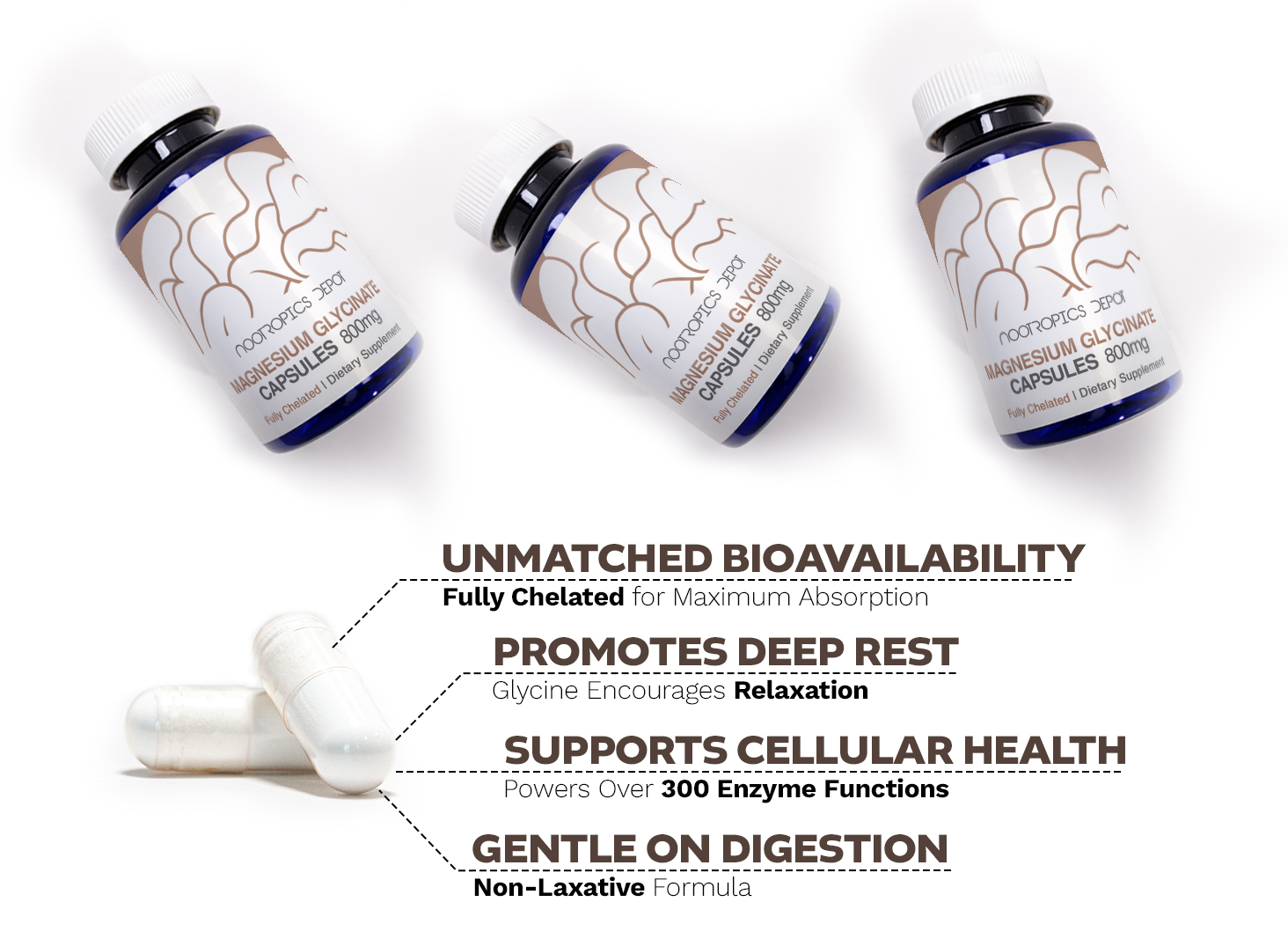
At Nootropics Depot, we're proud to offer our high-quality Magnesium Glycinate Capsules. Magnesium is a vital mineral involved in over 300 enzyme systems, playing a crucial role in numerous biological processes. However, many people struggle with magnesium deficiency due to poor absorption from typical supplements. Our Magnesium Glycinate Capsules address this issue by providing a highly bioavailable form of magnesium. Magnesium glycinate is a chelated form where magnesium is bound to the amino acid glycine. This unique structure enhances its absorption and utilization by the body. It's known for its gentle effect on the digestive system, making it an excellent choice for those who experience gastrointestinal discomfort with other magnesium forms. So, why should you take our Magnesium Glycinate Capsules? If you're looking to enhance your magnesium levels or support overall wellness, this form could be an excellent addition to your daily routine. Many of our customers have shared how it has helped them feel more energized and balanced, and we're confident you'll love it too!
Potential Benefits
Magnesium glycinate supplementation offers a range of potential benefits, thanks to its high bioavailability and the essential role magnesium plays in our bodies. One of the primary advantages is its support for muscle relaxation and nerve function. Many users report improved sleep quality and reduced muscle tension when taking magnesium glycinate regularly. This form of magnesium may also contribute to better stress management. Magnesium is involved in regulating the body's stress response, and adequate levels can help promote a sense of calm and relaxation. Some studies suggest that magnesium supplementation may help reduce anxiety symptoms and improve resilience to stressful situations. Magnesium glycinate may also support heart health by helping to regulate blood pressure and maintain a healthy heart rhythm. Additionally, it plays a crucial role in bone health by contributing to bone mineral density and calcium absorption. Cognitive function is another area where magnesium glycinate shows promise. Some research indicates that it may support various aspects of brain health, including memory, attention, and information processing speed. This could be due to magnesium's involvement in neurotransmitter function and its neuroprotective properties.

Quality and Standardization
At Nootropics Depot, we take immense pride in our rigorous sourcing and testing processes. Our Magnesium Glycinate undergoes thorough quality control checks to ensure purity and potency. We believe in transparency, which is why we provide detailed Certificates of Analysis (CoA) for each batch. Our commitment to quality extends beyond just testing. We carefully select the best raw materials and use state-of-the-art manufacturing processes to ensure that every capsule meets our high standards. This attention to detail is what sets us apart and allows us to offer you a superior magnesium supplement. We adhere to Good Manufacturing Practices (GMP) and our facilities are regularly inspected to maintain the highest quality standards. Our team of experts, including chemists and nutritionists, work tirelessly to ensure that our Magnesium Glycinate Capsules deliver consistent potency and purity. By choosing Nootropics Depot's Magnesium Glycinate, you're not just getting a supplement – you're getting a product backed by rigorous scientific standards and a commitment to excellence. Magnesium Glycinate vs. L-Threonate vs. Oxide: Which Is The Best Magnesium Supplement?
Mechanism of Action
Magnesium glycinate's effectiveness lies in its unique structure and absorption mechanism. The glycine component acts as a carrier for magnesium, enhancing its bioavailability. This form is absorbed in part as an intact dipeptide in the proximal small intestine, likely via a dipeptide transport pathway. Glycine, an inhibitory neurotransmitter, may contribute to the calming effects often reported with magnesium glycinate supplementation. It acts as an excellent pH buffer, significantly reducing the pH increase at tight junctions that typically hinders magnesium absorption. Once absorbed, magnesium plays crucial roles in numerous physiological processes. It acts as a cofactor in over 300 enzyme systems, regulating diverse biochemical reactions including protein synthesis, muscle and nerve function, blood glucose control, and blood pressure regulation. Magnesium is also essential for energy production, participating in both oxidative phosphorylation and glycolysis. It contributes to the structural development of bone and is required for the synthesis of DNA, RNA, and the antioxidant glutathione. The 5 Best Sleep Supplements That Aren't Melatonin
Overview Of The Science
Research on magnesium glycinate has shown promising results across various areas of health and wellness. Studies suggest that this highly bioavailable form of magnesium may offer benefits for sleep quality, stress management, and cognitive function. Several clinical trials have demonstrated that magnesium supplementation can improve sleep parameters, including sleep efficiency and onset latency. This is particularly notable in older adults and individuals with sleep disturbances. The mechanism appears to be related to magnesium's role in regulating neurotransmitters and melatonin production. In terms of stress management, magnesium glycinate has been shown to help regulate stress hormones and support the body's stress response. Some studies have found that magnesium supplementation may reduce anxiety symptoms and improve resilience to stressful situations. Cognitive function is another area where magnesium glycinate shows potential. Research indicates that it may support various aspects of brain health, including memory, attention, and information processing speed. This could be due to magnesium's involvement in neurotransmitter function and its neuroprotective properties. While magnesium glycinate shows promise on its own, some studies suggest that combining it with other supplements may offer synergistic benefits. For instance, pairing it with Bacopa Monnieri might enhance cognitive performance, while combining it with L-Theanine could provide additional support for stress management and relaxation.
Click here to read the Research Snapshot

Lab Testing Results / COAs
Trust Through Transparency
Your health matters, which is why we only use the best 3rd party ISO accredited labs and cGMP certified and FDA registered facilities right here in the USA
Learn more